The first and only 522 study to compare a single incision sling to full-length retropubic and transobturator slings
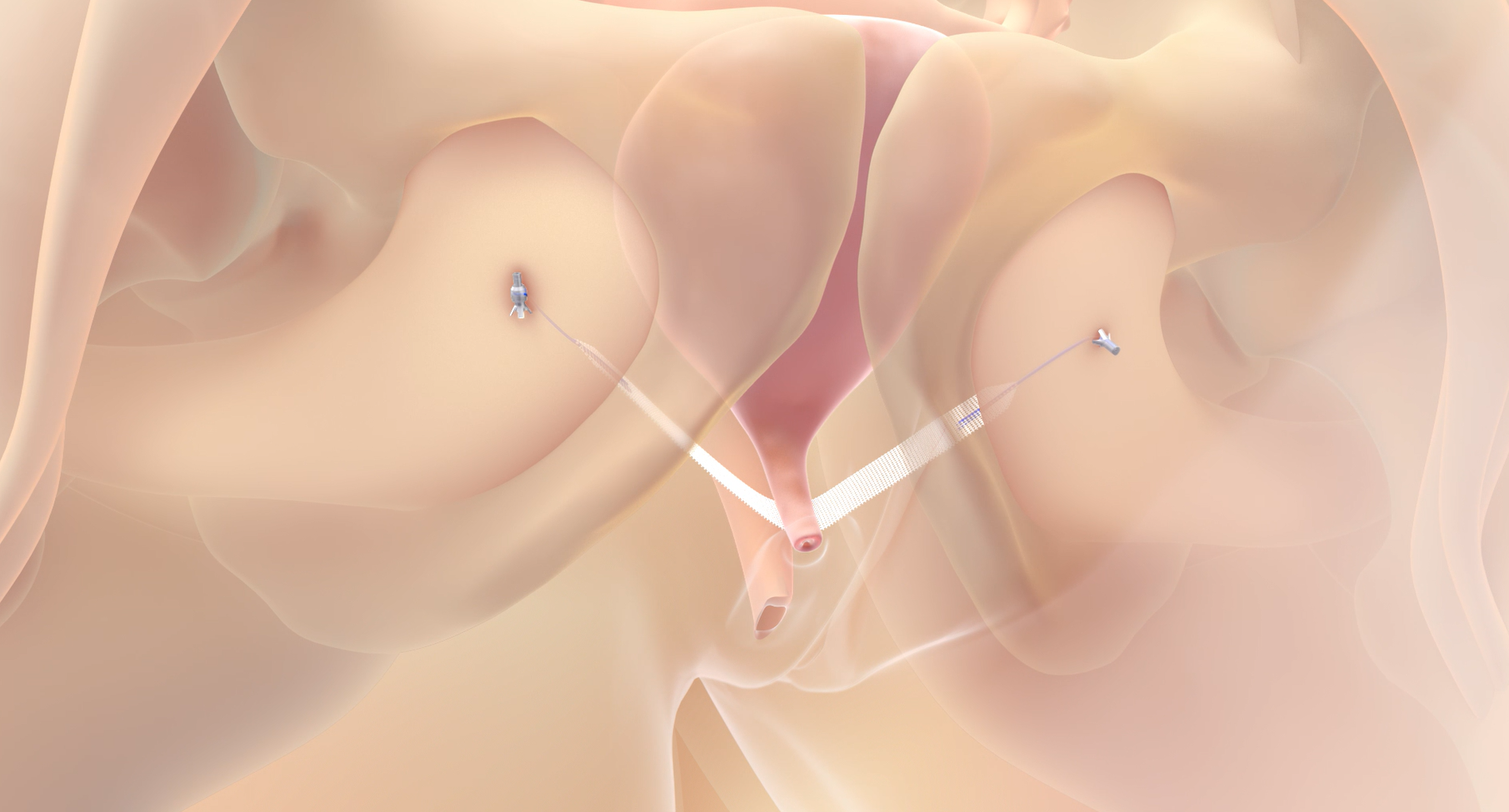
The Altis® 522 study is a prospective, post-market, multi-center, comparative cohort study performed in adult female patients with SUI who are clinically indicated for mid-urethral sling surgery.
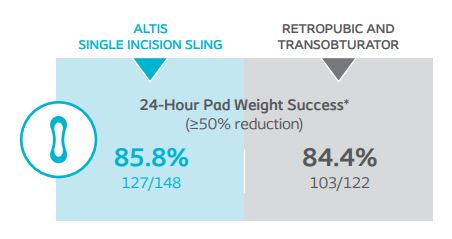
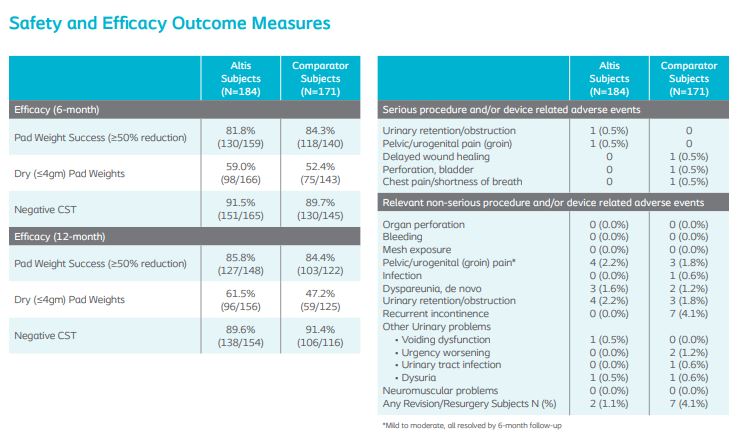
At 12-months, 24-hour pad weight success (≥50% reduction), negative CST, PGI-I, UDI-6, and IIQ-7 were similar between Altis and full-length retropubic and transobturator slings. Altis product design (i.e. elastic properties similar to the pubourethral ligament and intraoperative adjustability post-deployment) and surgical reproducibility attributed to high objective and subjective cure rates at 12-months, comparable to full-length retropubic and transobturator slings.

The Altis 522 study was designed to have a full range of objective and subjective outcome measurements, included full-length retropubic and transobturator slings in the comparator arm, and did not exclude ISD patients. The same rigorous and robust protocol and testing methods utilized in Altis IDE study were included in the Altis 522 study.

All commercially available full-length retropubic and transobturator slings were allowed in the comparator group.
By allowing a heterogenous group of full-length retropubic and transobturator slings in the comparator arm, and enrolling patients with a variety of characteristics, the Altis 522 study is more reflective of real world clinical practices.

The aim of the Altis 522 study is to compare the safety and effectiveness of the Altis Single Incision Sling to full-length retropubic and transobturator slings through 36-months. Subsequent reports will follow at 24- and 36-months.

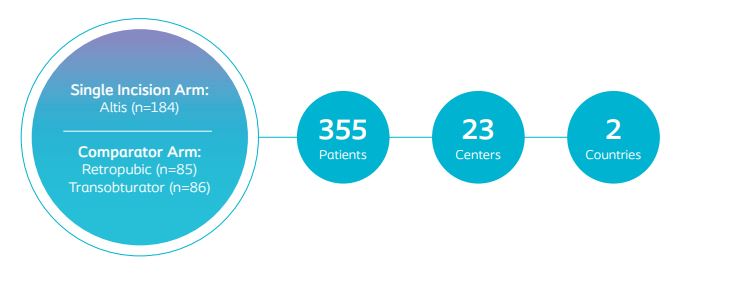
Primary Effectiveness Objective
Demonstrate that the reduction in 24-hour pad weight at baseline for Altis is non-inferior to full-length retropubic and transobturator slings.
Secondary Effectiveness Objective
Cough Stress Test (CST) and objective dryness (defined as ≤4.0gm pad weight).
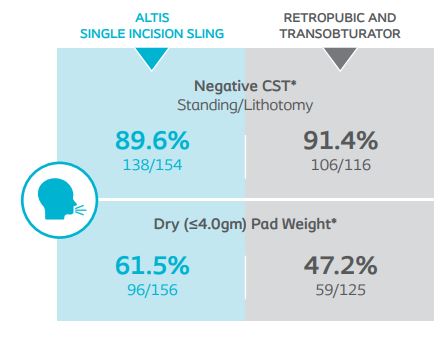
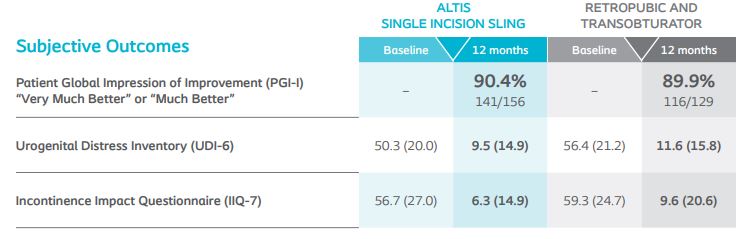
*Results compared to baseline collected at 12-months
Indications
The Altis Single Incision Sling Systems are indicated for the treatment of female stress urinary incontinence (SUI) resulting from urethral hypermobility and/or intrinsic sphincter deficiency (ISD).
Contraindications
Altis slings should not be used in pregnant women, or in women planning future pregnancies or any patient with potential for future growth. Altis slings should not be used in those who are taking anticoagulant therapy, who have an abnormal urethra, who have an intra-operative urethral injury, are immunocompromised, who have a sensitivity to polypropylene, have a pre-existing local or systemic infection, who are immunocompromised, or women that have a known or suspected pathology which would compromise implant or implant placement.
Warnings and Precautions
It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the possible warnings and precautions associated with the use of this product and the associated surgical risks. The Altis Sling Systems should only be used by surgeons familiar with the surgical procedures and techniques involving transvaginal placement of non-absorbable meshes and who have adequate education and experience in the treatment of female SUI. Based on physician experience and education, a thorough assessment of each patient should be made to determine the suitability of a synthetic mesh procedure. The patient should be counseled that alternative non-mesh incontinence surgeries may be appropriate, and the reason for choosing a mesh procedure should be explained. Physician should obtain patient consent prior to surgery and ensure that the patient has an understanding of the postoperative risks and potential complications of transvaginal mesh surgery. Patient counseling should include a discussion that the mesh to be implanted is a permanent implant, and that some complications associated with the implanted mesh may require additional surgery; repeat surgery may not resolve these complications. Serious adverse tissue responses or infection may require removal of mesh.
Adverse Effects
Potential adverse events are those associated with surgery using implantable synthetic mesh materials. As with all foreign bodies, the Altis sling is likely to exacerbate any existing infection. Local irritation at the wound site and/or a foreign body response may occur. The following complications are known to occur with synthetic mesh implantation: mesh erosion (e.g., vaginal, bladder), mesh extrusion, mesh exposure, infection, pain (acute or chronic), and bladder, bowel, urethra, vagina, vessel, and/or nerve perforation/injury. There is also the risk of complete failure of the procedure resulting in continued incontinence due to incomplete support or overactive bladder. The occurrence of these events may require partial or complete removal of the sling. Patients should be monitored regularly after the device has been implanted for immediate treatment of any adverse reaction.
See the device instructions for use for detailed information regarding the implant procedure, warnings /precautions, adverse reactions, prior to using this product.

- Happy Valentine’s from Andy Barham, Patient Educator
- Why should you always have an Elefant in the room? Discover the new Coloplast website
- The first and only 522 study to compare a single incision sling to full-length retropubic and transobturator slings
- Treating kidney stones within the kidney itself
-
Learn more about our Women’s Health portfolio and the experiences of your colleagues in the Netherlands and Belgium
Virtually, February 3 2022 -
115th Congress of the AFU 2021
Havane Bordeau, November 17 2021 -
Online Kiel School of Penile Surgery Registration is OPEN FROM NOW
Virtually, November 29 2021







